Overview
Levolta Pharmaceuticals’ mission is to overcome current treatment barriers by reformulating late-stage products, resulting in significantly less development costs and an enhanced therapeutic index.
Problem:
The current problem within the pharmaceutical industry is two-fold; 1) new drug development is lengthy and very expensive, leading to 2) a lack of effective new therapies to treat the ailments of consumers. Pharmaceutical development is risky due to the high upfront costs to develop, test and release new products. Many potential products fail in the clinical trials stage, or do not gain FDA approval, causing companies to absorb the sunk costs associated with product development.
The pharmaceutical industry continues to experience a high degree of clinical development failures and corresponding costs, which drive escalating consumer costs upon product approval. These risks have led to big pharmaceutical companies scaling down on R&D investments and evaluating business development opportunities for portfolio growth. The Generic industry is also experiencing risk, as profit margins have sunk to all-time lows which has led to M&A’s with “Ethical / Branded” pharmaceutical companies.
Solution:
To overcome these problems inherent in the industry, Levolta has created a re-formulation platform to enhance a product’s probability of success (testing and development), provide an enhanced therapeutic index and significantly reduce the development time lines by using 505(b)(2) regulatory filings, thus decreasing time lines by 8-10 years.
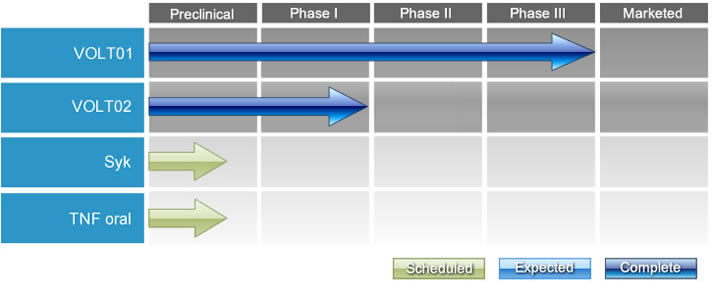
Our Pipeline:
VOLT01 (Zoledronic Acid Derivative)
Bisphosphonates (BP) are a class of drugs that prevent the loss of bone mass and are used in bone-related diseases such as osteoporosis, osteoarthritis, Paget’s disease and bone cancers. The most effective BP is Zoledronic Acid (ZA), which is given intravenously once a year. While very effective, ZA has two major side effects:
1) 44% of treated patients get flushing, fever, joint pains, and muscle aches, a syndrome called postdose syndrome (PDS).
2) More importantly, and rarely, patients get a serious condition called osteonecrosis of the jaw (ONJ).
Levolta’s derivative of Zoledronic Acid (VOLT01) was developed to improve both the efficacy and side effect profile vs ZA. Since VOLT01 is a combination of two approved marketed drugs, pre-clinical safety and toxicology studies are not required.
A randomized phase II trial comparing VOLT01 to ZA has been successfully completed. Based on the results, the clinical development of VOLT01 has moved into a phase III registration trial for patients with knee osteoarthritis.
VOLT02 (conjugated progesterone)
Progesterone is a steroid hormone involved in the female menstrual cycle, pregnancy and embryogenesis of humans and other species. While predominantly used in hormone-related care, it has been shown in phase II studies to be effective in the treatment of traumatic brain injury (TBI).
Despite its efficacy, progesterone suffers from a serious drawback in that it is water insoluble and may take up to 1.5 hours to get a single dose into solution.
VOLT02 improves water solubility dramatically and does not require pre-clinical toxicology or safety pharmacology studies.
Spleen tyrosine kinase (Syk) inhibitors
Syk is an important mediator of immunoreceptor signaling in macrophages, B cells, mast cells and neutrophils, and also plays a role in signaling in synoviocytes and osteoclasts. Levolta is particularly interested in its role in neutrophil and mast cell signaling. Syk is involved in activation of neutrophils through integrins and immunoreceptors. Neutrophils comprise 60% of all white blood cells and are responsible for mediating the early phases of the inflammatory response.
Under natural circumstances, neutrophils are recruited to the site of inflammation by mediators such as cytokines and complement. They undergo degranulation and respiratory burst, releasing granule contents, reactive oxygen intermediates and nitric oxide, thereby killing the invading bacteria.
Inhibiting Syk kinase will decrease immune cell activation. Blocking excessive activation and release of granule and respiratory burst products will inhibit the destruction seen in neutrophil mediated diseases such as vasculitis.
Levolta is developing inhibitors of Syk and through high throughput screening, has identified 9 compounds which target this enzyme. Pharmacology studies have revealed two clear leads for this program, which are ready for lead optimization studies. By targeting only a portion of the immune system, we expect a much better safety profile than current treatments.
Tumor Necrosis Factor (TNF)
The most popular biologics for inflammatory diseases are the antibody based anti-TNFs. These have been approved for the treatment of rheumatoid arthritis, psoriasis, ankylosing spondylitis, Crohn’s Disease, psoriasis and psoriatic arthritis. Biologics have shown good efficacy in those diseases in which they work but suffer from safety issues and patient preference for oral drugs. In addition, they are expensive to develop and to manufacture. These costs are transferred to the health care system and are expected to rise over the next 5 years.
Levolta is developing new non-biologics and will capitalize on the above mentioned weaknesses of currently marketed anti-TNFs. Our compounds are small molecules thus manufacturing will be much less expensive than biologics. Small molecules can be formulated as an oral therapy and as these compounds will be competing against costly biologics, there will be strong payer support for these drugs.
Levolta has identified 4 compounds which inhibit TNF.